CNS and neurodegenerative diseases
Diseases and traumas in Central Nervous System blood vessels and neurons lead to a wide range of neurodegenerative conditions. The study of the μ-Vascular Network (VN) and Neuron Network (NN) organization is a crucial physiological issue, for which quantitative structural measurements are a key step.
In clinical framework, there is growing interest in high-resolution imaging techniques, allowing for a direct, quantitative estimation of important morphological and topological parameters characterizing the VNs and neuronal morphology for the investigation of several pathologies. However, current imaging tools do not generate sufficient dimensionality, resolution, contrast and other factors critical to investigate neurodegenerative pathologies and spinal-cord-injuries, as well as to understand the relationship between vascular and neuronal systems.
By using Synchrotron X-Ray Phase Contrast Microtomography (XPCμT), we be able to achieve high image contrast and high-resolution 3D visualization of ex vivo mouse spinal cord microvascular and neuron network in the same image.
Anomalous development or damage to the vascular network (VN) of the central nervous system (CNS), as well as impaired partnership with neurons and glia, are related to many serious pathologies.
A simultaneous study of the complexity of the VN and Neurons with a 3D high resolution image in order to discriminate the smallest capillaries and the neuron morphology is crucial.
To this aim, synchrotron X ray phase contrast micro tomography (XPCμT) technique achieves high image contrast and enables high-resolution 3D visualization of low absorbing system.
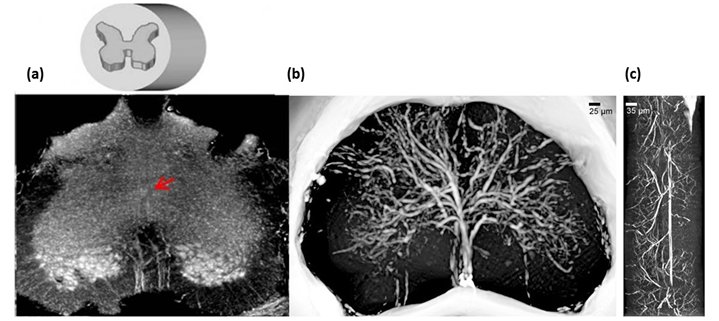
By using High resolution XPCμT at beamline ID17, at European Synchrotron Radiation Facility (ESRF) and at TOMCAT, at Paul Scherrer Institut (SLS-PSI), we have been able to visualise the complete architecture of both the vascular network and the neuronal system of the mouse spinal cord (Figure 1).
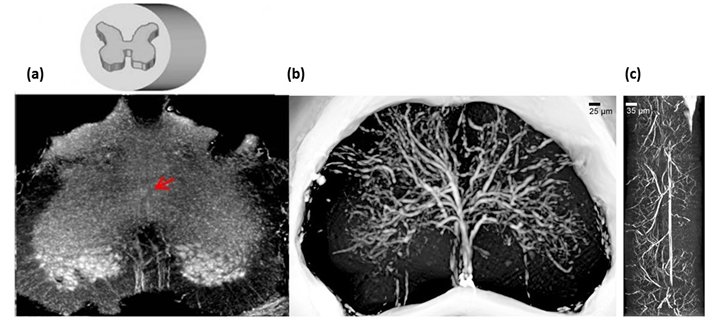
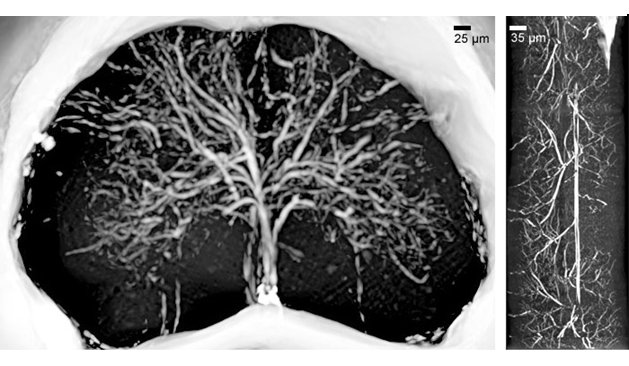
Figure 1: (a) X-ray phase contrast microtomography of the lumbar-sacral region of the spinal cord (size: 1.2 mm). (b) Reconstructed volume of the lumbar-sacral region aft er administration of the MICROFIL contrast agent. The image pixel size is 3.5 μm. (c) Longitudinal view of (b), 1 mm long.
We imaged single elements of the networks such as microcapillaries and micrometric nerve fibres, axon-bundles and neuron soma (figure 2). This imaging was carried out without sectioning or other destructive sample preparation procedures [1].
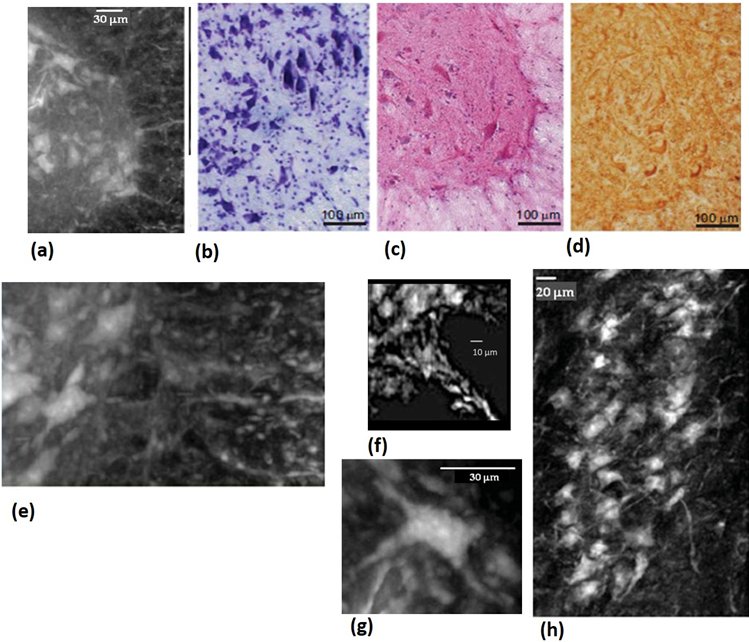
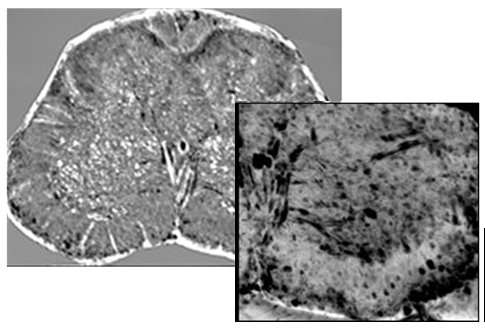
Figure 2: Neural population: (a) White/grey matter interface of a thick slab selected in the anterior horn of the lumbar-sacral spinal cord. (b,c,d) For comparison, microscope histological images: (b) Histology (Nissl staining), (c) Histology (He/eo staining) and (d) Immunohistochemical analysis of SMI-32, a marker of motor neurons. (e) zoom of the with/grey matter anterior horn interface. (f) Magnification of a single neuronal cell. (g) Image of one nerve fibre at the interface with the grey matter. (h) Longitudinal view (length: 0.5 mm) of the sample at the same interface.
The high quality of the obtained images enables a quantitative study allowing the extraction of relevant geometrical information about the neuronal structure on a subject by subject basis. We developed and applied a spatial statistical analysis on neurons to obtain quantitative information on the 3D arrangement of neural cells. Our approach paves the way to the creation of a database for the characterization of the main features of neuronal network and for a comparative investigation of neurodegenerative diseases and therapies. Our work focuses on an interdisciplinary research, we engage biomedical science and physics with the intent to focus on the translation of modern X-ray physics concepts to pre-clinical applications. In addition, we demonstrates the potentialities of the XPCμT showing that it can be applied to a large number of ex vivo pre-clinical studies.
References
[1] M. Fratini et al Scientific Reports 5, 8514 (2015)
Collaborators
ID 17, European Synchrotron Radiation Facility, ESRF, Grenoble, France
TOMCAT, Swiss Light Source, Paul Scherrer Institut, Villigen, & Centre d'Imagerie BioMedicale, Ecole Polytechnique Federale de Lausanne (Switzerland)
SYRMEP, Elettra - Sincrotrone Trieste S.C.p.A. (Italy)
Institute of Crystallography - CNR, Monterotondo, Rome (Italy)
Department of Experimental Medicine, University of Genova & AUO San Martino - IST Istituto Nazionale per la Ricerca sul Cancro, Genova (Italy)
"Enrico Fermi" Centre MARBILab c/o Fondazione Santa Lucia, Rome (Italy)
I.R.C.C.S. Neuromed, Pozzilli (Italy)
Department of Physics, Sapienza University, Rome (Italy).
Degenerations in vascular and neuronal networks of the spinal cord affected by multiple sclerosis (MS) are currently the object of intensive investigation. The use of animal models such as experimental autoimmune encephalomyelitis (EAE) facilitates the exploration of new treatments. EAE, an experimental model for MS, is an autoimmune disease of the CNS driven by autoreactive myelin-specific T cells that infiltrate through an impaired blood-brain barrier (BBB) leading to neuroinflammation. The inflammation is characterized by microgliosis and astrogliosis and results at the neuropathological level in demyelination and irreversible axonal loss, which account for neurological impairment.
At present, there is considerable interest in the innovative approach of using cell-based therapies, which may provide support for CNS repair through their neuroprotective effect [1] in MS.
Mesenchymal stem cells (MSC) are being considered as an alternative therapeutic approach in neurological diseases such as MS, in view of their potential regulatory effect on immune cells, in particular their immunosuppression of T cells and their inhibition of dendritic cell maturation, as well as their neuroprotective features.
Preclinical studies have demonstrated the benefit of treatment with MSC on EAE model, with reduction of clinical severity, together with histopathological data indicating decreased demyelination and axonal loss. However, lesions in EAE and the role of MSC are still debated. We use X-ray Phase-Contrast Tomography to investigate 3D sub-micron damage in the Vascular and Neuronal Networks (VN and NN) in EAE with and without MSC treatment.
Conventional techniques entail sample preparation and sectioning which condition the results, while we provide an unprecedented direct 3D morphological description of EAE lesions.
We clearly detected a deficit in the VN, including capillaries and vessels at disease onset and a reduction in the VN through MSC treatment (Figure 1). Similarly, the attack to myelin and neurons also appears to be reduced by MSC administration.
This study paves the way to a more advanced study of immune-mediated CNS diseases and to more accurate monitoring of treatment effectiveness.
(a) X-ray phase contrast microtomography of the lumbar-sacral region of the spinal cord (size: 1.2 mm). (b) Reconstructed volume of the lumbar-sacral region aft er administration of the MICROFIL contrast agent. The image pixel size is 3.5 μm. (c) Longitudinal view of (b), 1 mm long.
References
[1] Uccelli, A., L. Moretta and V. Pistoia (2008). Mesenchymal stem cells in health and disease. Nat Rev Immunol 8(9): 726-736.
Collaborators
ID 17, European Synchrotron Radiation Facility, ESRF, Grenoble, France
Institute of Crystallography - CNR, Monterotondo, Rome (Italy)
Department of Experimental Medicine, University of Genova